LSU Professor Receives NIH Funding for Glycoconjugate Vaccine Research to Combat Drug-Resistant Infections
September 30, 2024
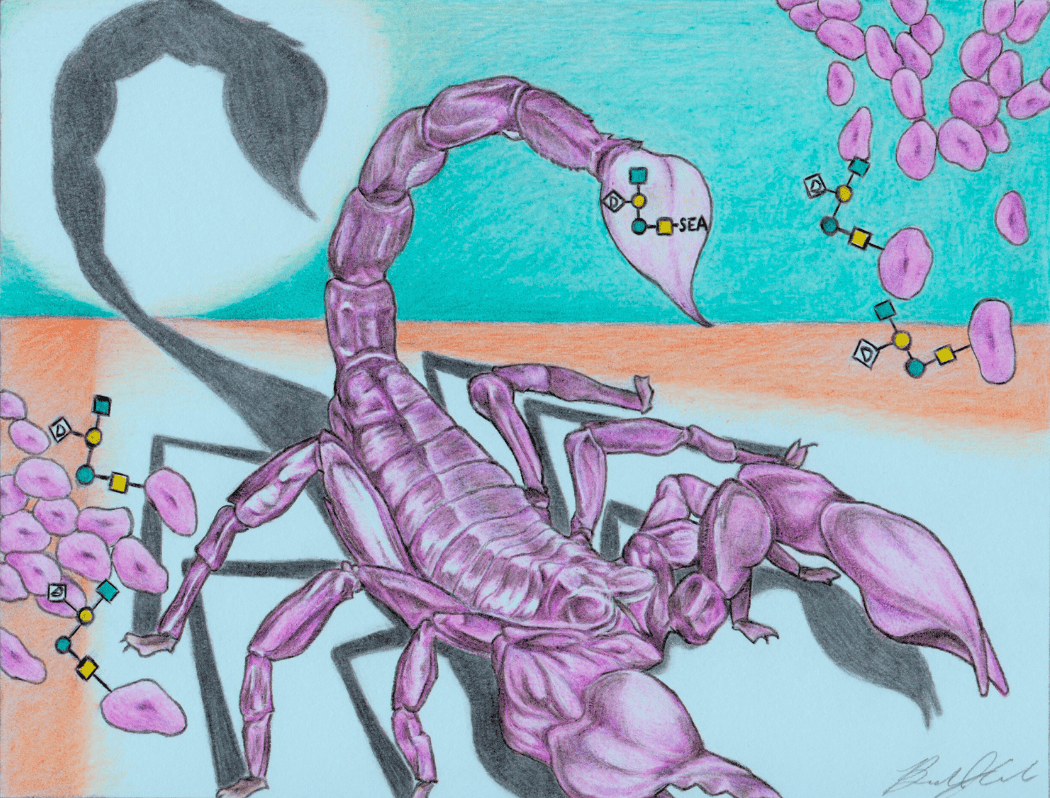
Cover art illustrating a scorpion amongst a colony of A. baumannii bacteria. The bacteria bears the target pentasaccharide, depicted using a “sugar code” notation on the scorpion′s stinger, symbolizing the pentasaccharide's role in virulence during A. baumannii infection. LSU Chemistry alumnus Brandon Conrad (PhD 2024) designed the cover.
– Total Synthesis of a Pentasaccharide O‐Glycan from Acinetobacter baumannii (Eur. J. Org. Chem. 16/2023), Copyright © 2023, John Wiley and Sons
Professor Justin Ragains from the LSU Department of Chemistry received a grant from the National Institutes of Health, or NIH, to develop a novel glycoconjugate vaccine aimed at preventing infections caused by Acinetobacter baumannii, a pathogen responsible for severe hospital-acquired and combat-related infections that often lead to critical illness and high mortality rates.
With the growing prevalence of drug-resistant strains and the lack of an approved vaccine for A. baumannii, there is an urgent need for innovative strategies for the prevention and treatment of infections associated with this bacterium. Ragains' project aims to synthesize and evaluate glycoconjugates that trigger immune responses, protecting against A. baumannii infections.
“Vaccines based on the covalent conjugation of glycans to carrier proteins have been successful in preventing infections associated with other bacterial pathogens, such as Haemophilus influenzae and Streptococcus pneumoniae,” Ragains said. “We aim to follow a similar approach but with two ‘non-traditional’ classes of surface glycans from A. baumannii that we will conjugate to carrier proteins for vaccine development.”
In A. baumannii, lipooligosaccharides, or LOS, are integral to the bacteria's ability to invade, replicate, and persist in host cells. At the same time, pentasaccharide O-glycans are vital for protein stability, immune signaling, and cell-to-cell communication. By incorporating these two surface glycans into vaccine development, Ragains hopes to harness their immune-modulating properties and create an effective vaccine that protects against multiple strains.
In a previous study, the Ragains research group chemically synthesized a pentasaccharide O-glycan from A. baumannii, creating a homogeneous and conjugation-ready glycan (Nieri & Ragains, 2022). The team will now synthesize LOS fragments and then conjugate the two classes of glycans to carrier proteins, in collaboration with Andrew Lees and his team at Fina Biosolutions in Rockville, MD.
Using an animal model, Ragains will conduct immunological studies in collaboration with Associate Professor Hong Xin at the LSU Health Sciences Center, New Orleans. “The animal studies will provide essential insights into the efficacy and safety of the binary glycoconjugate vaccine in preventing A. baumannii infections,” Ragains said. “By testing the immune response and protection levels in these models, we can advance the development of a vaccine that targets antibiotic-resistant strains, paving the way for a formulation that can be adapted for human use.”
For more information about Professor Ragains' research and his group’s ongoing efforts to combat drug-resistant infections caused by Acinetobacter baumannii, visit the Ragains research group webpage.